
As the name suggests, most electronic devices today operate through the movement of electrons. But materials that can efficiently conduct protons, the nuclei of hydrogen atoms, could be key to several important technologies to combat global climate change.
Most proton-conducting inorganic materials currently available require undesirably high temperatures to achieve sufficiently high conductivities. However, lower-temperature alternatives could enable a range of technologies, including more efficient and durable fuel cells that produce clean electricity from hydrogen, electrolyzers that create clean fuels like hydrogen for transportation, solid-state proton batteries, and even new types of computing devices based on the ion-electron effect.
MIT engineers have identified specific properties of materials that lead to fast proton conduction, in an effort to advance the development of proton conductors. Using these properties quantitatively, the team has identified six new candidates that appear promising as fast proton conductors. Simulations show that these candidates will perform significantly better than existing materials, but they still need to be experimentally validated. In addition to discovering potential new materials, the research provides a deeper understanding of how these materials work at the atomic level.
The new findings are described in the journal. Energy and Environmental SciencesIn a paper written by MIT professors Bilge Yildiz and Ju Li, postdocs Pjotrs Zguns and Konstantin Klyukin, and their collaborator Sossina Haile and her students at Northwestern University, Yildiz is the Breene M. Kerr Professor in the Department of Nuclear Science and Engineering and the Department of Materials Science and Engineering.
“Proton conductors are needed for clean energy conversion applications such as fuel cells, which use hydrogen to produce carbon dioxide-free electricity,” Yildiz explains. “We want to do this process efficiently, so we need materials that can transport protons through these devices very quickly.”
Current methods of producing hydrogen, such as steam methane reforming, emit large amounts of carbon dioxide. “One way to get around that is to electrochemically produce hydrogen from steam, which requires a very good proton conductor,” says Yildiz. The production of other important industrial chemicals, such as ammonia, and potential fuels, could also be accomplished through efficient electrochemical systems that also require good proton conductors.
But most inorganic materials that conduct protons can only operate at temperatures between 200 and 600 degrees Celsius (450 and 1,100 degrees Fahrenheit) or higher. These temperatures require energy to maintain and can cause the material to deteriorate. “You don’t want to go to higher temperatures because it makes the whole system more difficult, and the durability of the material becomes an issue,” Yildiz says. “There are no good inorganic proton conductors at room temperature.” The only known room-temperature proton conductors are polymer materials, which she says are not practical for computing devices because they cannot be easily scaled down to the nanometer level.
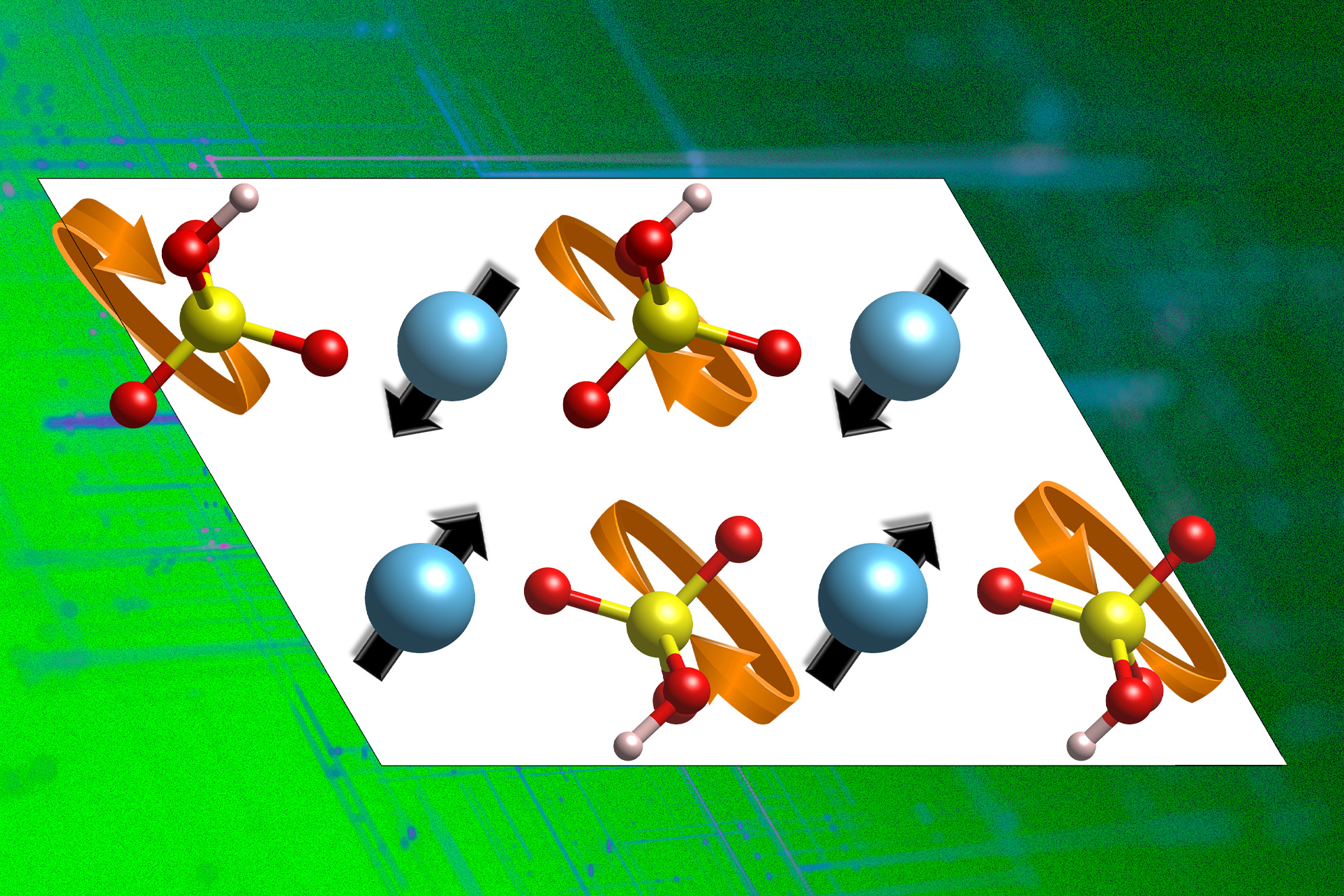
To solve the problem, the team first had to develop a fundamental, quantitative understanding of exactly how proton conduction works, taking a class of inorganic proton conductors called solid acids. “We first need to understand what governs proton conduction in these inorganic compounds,” she says. While looking at the atomic composition of the materials, the researchers identified two properties that are directly related to the material’s proton-carrying potential.
As Yildiz explains, proton conduction first involves a proton “jumping from the donor oxygen atom to the acceptor oxygen.” Then the environment must reorganize to remove the accepted proton, which then jumps to another neighboring acceptor, allowing long-distance proton diffusion. This process occurs in many inorganic solids, she says. Figuring out how the last part works—how the atomic lattice reorganizes to dislodge the accepted proton from its original donor atom—is a key part of the research, she says.
Researchers used computer simulations to study a class of materials called solid acids that are good proton conductors at 200 and above. Celsius. This class of materials has a substructure called a polyanion group sublattice, which must rotate to allow protons to be removed from their original location and transferred to another location. The researchers were able to identify the phonons that contribute to the flexibility of this sublattice, which is essential for proton conduction. They then used this information to comb through a vast database of theoretically and experimentally possible compounds to find better proton conducting materials.
As a result, they found solid acid compounds that were promising proton conductors and had been developed and produced for a variety of other applications but had never been studied as proton conductors. These compounds turned out to have the right properties of lattice flexibility. The team then performed computer simulations of how certain materials identified in the initial screening would perform at relevant temperatures to confirm their suitability as proton conductors for fuel cells or other applications. Sure enough, they found six promising materials whose predicted proton conduction rates were faster than the best existing solid acid proton conductors.
“There are uncertainties in these simulations,” Yildiz cautions. “I don’t want to say exactly how much higher the conductivity will be, but these look very promising. I hope this will motivate the experimental field to synthesize these in different forms and use these compounds as proton conductors.”
It could take years to translate these theoretical findings into practical devices, she says. The first applications, she says, are likely to be electrochemical cells that produce fuels and chemical feedstocks, such as hydrogen and ammonia.
This research was supported by the U.S. Department of Energy, the Wallenberg Foundation, and the National Science Foundation.


